Main Projects
Circulating Tumor Cells
Microtentacles
Metastasis remains the leading cause of death among cancer patients. Circulating tumor cells (CTCs) pose a particular challenge as our knowledge of the interaction between CTCs and the circulatory microenvironment is not well-defined. It is now understood that tipping the cytoskeletal force balance toward microtubule stabilization and actin depolymerization causes CTCs to acquire characteristics that promote metastatic efficiency. Through stabilization by detachment-induced post-translational modifications and interactions with accessory proteins, microtubules can overcome the restrictive forces of cortical actin to protrude the plasma membrane; thus mediating microtentacle (McTN) formation, cell-cell aggregation and endothelial attachment of CTCs.

Stabilizing drugs, like taxanes, prevent microtubule disassembly, stalling tumor cells in metaphase and initiating cell death through spindle checkpoint activation or mitotic catastrophe. While microtubule-targeted drugs are highly successful in primary tumor treatment, their success is limited for metastatic disease. The effects of microtubule-targeted chemotherapies on CTCs have not been thoroughly investigated. Evidence that taxanes enhance McTN formation and tumor cell reattachment emphasizes that the effects of existing cancer drugs on CTCs should be examined to ensure that anti-mitotic therapies do not inadvertently increase metastatic potential.
Considering the detrimental effects of chemotherapeutics on CTC mobilization and McTN induction, it raises the question of whether our anti-cancer effort is missing an important target. It remains critical to develop methods that distinguish whether drug-induced tumor shrinkage arises from tumor cell death or tumor cell dissemination, since these two effects have dramatically different consequences in a neoadjuvant setting. While current drug development efforts and clinical endpoints focus on limiting the growth of either primary or metastatic tumors, it will be important to understand the effects of existing cancer drugs on CTCs in order to improve treatment strategies and develop novel therapies aimed at reducing the metastatic potential of CTCs.

The Cytoskeleton and EMT
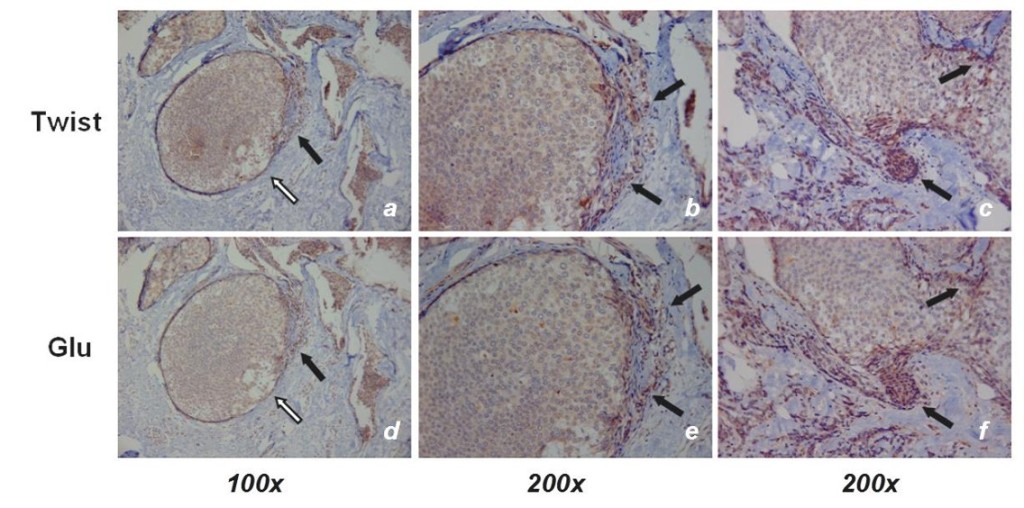
Epithelial-to-mesenchymal transition (EMT) is associated with increased breast tumor metastasis, but the specific mechanisms by which EMT promotes metastasis remain somewhat unclear. Specific post-translational α-tubulin modifications are critical for adherent cell motility and implicated in numerous pathologies, but also remain understudied in detached cells. We report here that EMT induced through ectopic expression of Twist or Snail promotes α-tubulin detyrosination and the formation of tubulin-based microtentacles in detached human mammary epithelial cells. Mechanistically, EMT downregulates tubulin tyrosine ligase enzyme resulting in an accumulation of detyrosinated α-tubulin (Glu-tubulin), and increases microtentacles that penetrate endothelial layers to facillitate tumor cell reattachment. Suppression of endogenous Twist in metastatic human breast tumor cells is capable of reducing both tubulin detyrosination and microtentacles.
Clinical breast tumor samples display high concordance between Glu-tubulin and Twist expression levels, emphasizing the coupling between EMT and tubulin detyrosination in vivo. Coordinated elevation of Twist and Glu-tubulin at invasive tumor fronts, particularly within ductal carcinoma in situ samples, establishes that EMT-induced tubulin detyrosination occurs at the earliest stages of tumor invasion. These data support a novel model where the EMT that occurs during tumor invasion downregulates tubulin tyrosine ligase, increasing α-tubulin detyrosination and promoting microtentacles which could enhance the reattachment of circulating tumor cells to the vascular endothelium during metastasis.
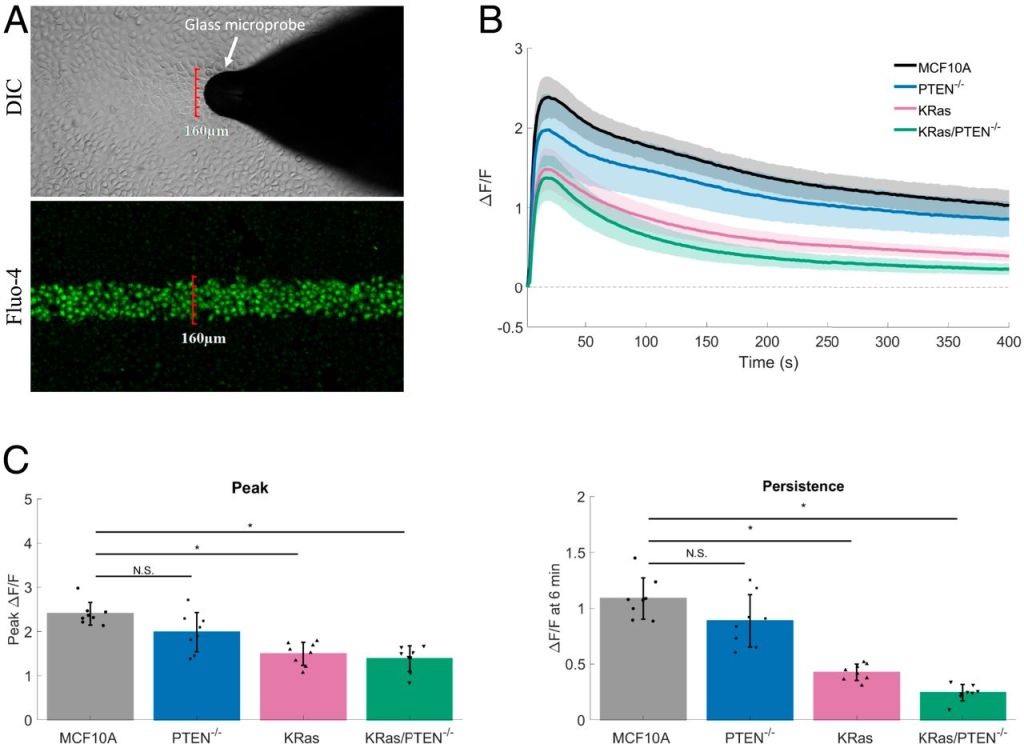
Calcium and Mechanotransduction
Changes in the mechanical microenvironment and mechanical signals
are observed during tumor progression, malignant transformation,
and metastasis. We report that normal breast epithelial cells are mechanically
sensitive, responding to transient mechanical stimuli through a
two-part calcium signaling mechanism. We observed an immediate,
robust rise in intracellular calcium (within seconds) followed by a persistent
extracellular calcium influx (up to 30 min). This persistent calcium
was sustained via microtubule-dependent mechanoactivation of
NADPH oxidase 2 (NOX2)-generated reactive oxygen species (ROS),
which acted on transient receptor potential cation channel subfamily
M member 8 (TRPM8) channels to prolong calcium signaling. In contrast,
the introduction of a constitutively active oncogenic KRas mutation
inhibited the magnitude of initial calcium signaling and
severely blunted persistent calcium influx. The identification that oncogenic
KRas suppresses mechanically-induced calcium at the level of
ROS provides a mechanism for how KRas could alter cell responses to
tumor microenvironment mechanics and may reveal chemotherapeutic
targets for cancer.
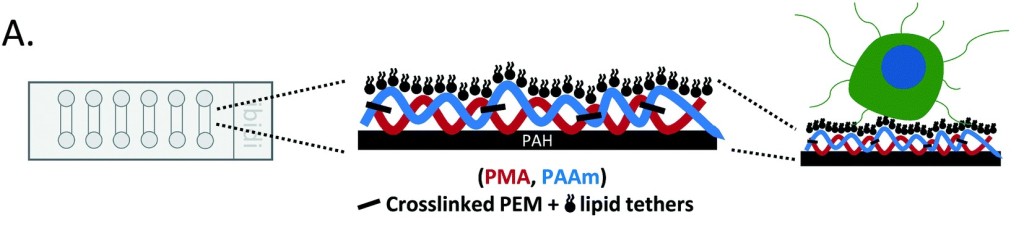
Tether-ChIP
We optimized a microfluidic device (TetherChip) engineered to prevent cell adhesion with an optically-clear, thermal-crosslinked polyelectrolyte multilayer nanosurface and a terminal lipid layer that simultaneously tethers the cell membrane for improved spatial immobilization. Thermal imidization of the TetherChip nanosurface on commercially-available microfluidic slides allows up to 98% of tumor cell capture by the lipid tethers. Importantly, time-lapse microscopy demonstrates that unique microtentacles on non-adherent tumor cells are rapidly destroyed during chemical fixation, but tethering microtentacles to the TetherChip surface efficiently preserves microtentacle structure post-fixation and post-blood isolation.